CROs are commissioned to conduct clinical trials for a variety of drugs and medical devices, and have been contributing to the development of new treatments. Currently, the development of regenerative medicine products has been becoming more and more active as a new development field. Remedy has also been engaging in the development (clinical trials and clinical researches) of various regenerative medicine products.
When you hear the word of "regenerative medicine," you may think of products using iPS cells or ES cells. Furthermore, clinical trials for regenerative medicine products are different from those for conventional drugs, and some people may have image that conducting the clinical trials seem challenging.
In this paper, we will explain "clinical trials for regenerative medicine products".
In Good Clinical Practice (GCP) which is an ordinance that must be adhered to in clinical trials, a guideline is established each for pharmaceuticals, medical devices and regenerative medicine products.
However, all the guidelines build on one fundamental objective, "ensuring the scientific quality and reliability of the results of clinical trials by placing the utmost importance on protecting the human rights, maintaining the safety and improving the welfare of subjects”.
No matter how scientifically or socially important it is, careful consideration must be given to eliminate the disadvantages of subjects.
Differences between pharmaceutical clinical trials and clinical trials for regenerative medicine products
What are specific differences between pharmaceutical clinical trials and those for regenerative medicine products?
As mentioned above, the basic objective remains the same for both types of the clinical trials. Therefore, there are not many major differences in the actual processes of the clinical trials.
This is because "regenerative medicine" refers to "products that treat or prevent diseases by using genes or cells" and does not necessarily refer to only regenerative medicine.
There are products using cells for the purpose of repairing tissues such as cartilage and skin, but those using gene vectors or transgenic cells to treat cancer are also categorized as regenerative medicine products.
However, there are a wide variety of specific clinical trial considerations about regenerative medicine products.
The first one is about the system.
Since regenerative medicine products use living cells and genes, it is necessary to strictly define the management system in the manufacturing and transportation processes as well as the system in the usage of the products at medical institutions.
Therefore, it is necessary to carefully check whether clinical trial sites are qualified to use the clinical trial products in consideration of the products’ features.
Even in a general pharmaceutical clinical trial, a survey to check the eligibility of clinical trial sites is conducted. However, expiration dates of many regenerative medicine products are only for two to three days. Therefore, it is required to carefully investigate the trial sites in light of the transportation route from the cell culturing processing facility (manufacturing plant) to the sites.
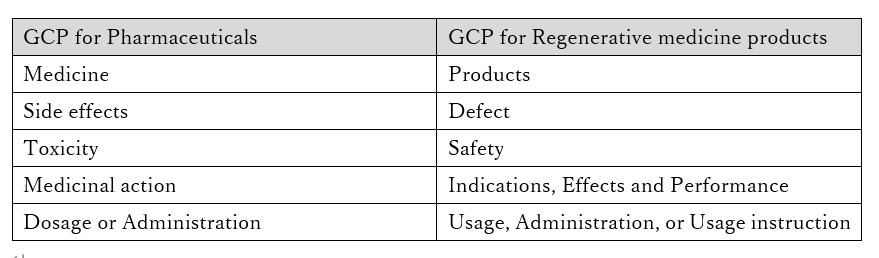
The next important difference is handling of adverse events (side effects).
In pharmaceutical clinical trials, we collect information on adverse events (side effects) of subjects for which a causal relationship with the investigational drug cannot be denied.
On the other hand, in clinical trials for regenerative medicine products, we check not only for adverse events but also for defects in the product itself, and collect these as "safety information”.
In the case of serious adverse events, such as death and hospitalization, an emergency report to the sponsor is usually required within 24 hours after receiving the information, and a report to the authorities by the deadline which is specified for each criterion of seriousness.
In the case of clinical trials for regenerative medicine products, even if adverse events don’t occur but it is recognized that there is a "risk" of a serious adverse event, an emergency report is required as is the case with occurrence of a serious adverse event.
In addition, there are a wide range of other matters that need to be confirmed depending on the characteristics of the product. In some cases, preparation methods when using the product need to be checked, and educational training for surgeries and procedures may also be required. In the case of combination products with drugs and medical devices, reporting methods may vary depending on the product.
Those who are engaged in the development of regenerative medicine products are required to have professional and scientific knowledge of cell culture and genetic engineering, as well as the ability to deliver these contents to various stakeholders including authorities, medical professionals and patients in an easy-to-understand manner.
Remedy Group provides our CRAs with further specialized training of regenerative medicine based on the knowledge of GCP.
With this training, we have been cultivating specialists who can adequately perform basic clinical trial tasks and handle clinical trials for regenerative medicine products as well, and we have been involved in the development of a wide variety of products.
If you have any questions about the development in the field of regenerative medicine products where the market is expected to further expand in the future, please contact us.