What image do you have of “Regenerative medicine”? Magic drug to remove nasolabial fold? Hair root regrowth or Cloned human?
Following are 6 meanings of “regeneration” written in a dictionary:
- Nearly dead creatures bring back to life, or making the creatures relive
- Reforming oneself and being back to a decent lifestyle
- Manufacturing products with waste materials
- Playing recorded tapes and discs with equipment
- Regenerating lost body parts
- 【Psychology】Recalling one’s memory or experiences
No. 1 and 2 could happen only in movies or TV dramas.
n the field of regenerative medicine, No. 5 is a proper meaning for “regeneration” indicating that damaged cells, tissues and organs are restored.
When you hear the word of “regeneration”, you may think of a lizard tail off the top of your head. When lizards are attacked by enemies, they fall off tails and escape from the enemies. However, their tails regenerate after a short time.
“Regenerative medicine” is a treatment to use this regenerative method and cure injuries and diseases. In other words, it is a treatment method to regenerate (or restore) cells, tissues and organs (In some cases, movement of genes) that have been damaged or lost by injuries, diseases or congenital issues. It can be said that the regenerative medicine therapy has a possibility to completely cure severe injuries and diseases previously treated only by symptomatic therapy.
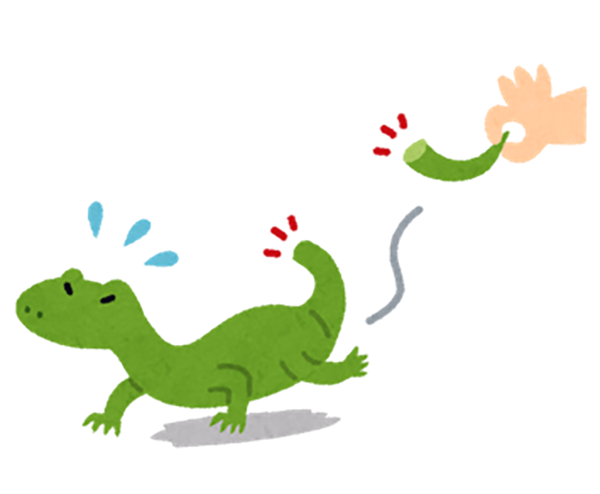
However, unlike lizards, unfortunately humans don’t have regenerative abilities. Liver is the only human organ that we can regenerate.
In the Greek mythology, there is a story like this: Prometheus stole a lightning bolt from Zeus to give it to humans. Zeus became furious at Prometheus. He ordered to Prometheus chained to a rock and had eagles keep nibbling at his body as punishment for stealing the lightning bolt. The eagles nibbled at his liver mostly. (His liver had regenerated over the night, and the eagles started nibbling at the regenerated liver again in the next morning.)
Considering the fact that the liver was kept being nibbled, early people might have realized the fact that other human organs except liver don’t have regenerative abilities. Therefore, humans have been feeling envy at the regenerative ability that lizards have from time immemorial and wishing if humans could regenerate body parts.
A long-time dream of humans “regeneration” became no longer a dream by the emergence of ES cells, iPS cells and genetic modification technology.
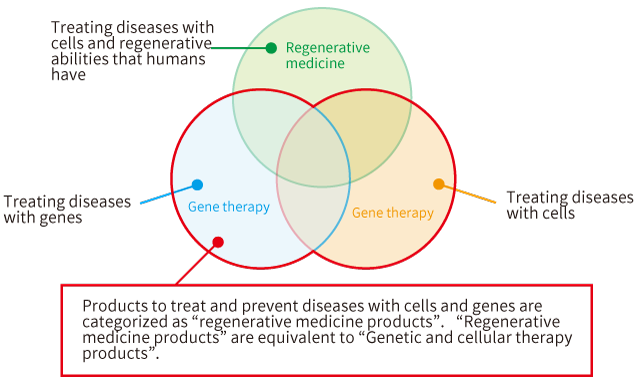
What is “regenerative medicine product”?
The Pharmaceutical Affairs Law, a law to regulate medical products, was renamed to the Pharmaceutical and Medical Device Act in 2014, and a new classification of “regenerative medicine product” was established aside from “medical product” and “medical device”. What is this regenerative medicine product?
Is it a product to be used in the field of regenerative medicine (restoring cells, tissues, organs and activities of genes)?
In the Pharmaceutical and Medical Device Act, the regenerative medicine product indicates “a product to treat or prevent diseases with genes and cells”, and it is not a product used in the field of regenerative medicine. In the law, a product to treat diseases with genes and cells and cannot be categorized as a medical product or medical device have been categorized as a regenerative medicine product. In Japan, regenerative medicine products are “genetic and cellular therapy products”.
For example, the aim of the cancer therapy is to kill cancer cells. It is not regenerative medicine with the aim of regenerating cells and tissues (It is not to restore cancer cells to normal cells.). However, cancer therapies using genes (oncolytic virus) and cells (CAR-T cells) are categorized as “regenerative medicinal product”.
In reverse, if a drug to promote regeneration is developed in the future and nerve cells are regenerated by the administration of the drug, this treatment will be categorized as “medical device”.
Also, the vascular regeneration therapy to leave tubular scaffolds for cells in a body of a patient and have vascular endothelial cells of the patient engraft into the inside wall of the tubular scaffolds has been developed in overseas.
In Japan, this therapy is categorized as “medical device”.
Since regenerative medicine products (genetic and cellular therapy products) are generated by live cells and virus, the development process, quality management, logistic and transportation ways and action mechanism are completely different from these of medical products and medical devices.
Therefore, when clinical trials for regenerative medicine products are conducted, it is required to form new manuals and structure for the regenerative medicine products. Also, those who take a part in the development of regenerative medicine products need to have specialized knowledge of cell culturing and gene engineering, scientific knowledge and capabilities to intelligibly convey necessary information of the clinical trials to the regulatory authorities, medical staff, patients and various stakeholders.
In order to have our CRAs acquire comprehensive knowledge of regenerative medicine and strengthen their explanation capabilities, intellim Corporation has been providing them with regenerative medicine specialist training and certification examination and striving to enhance development of CRAs who can lead the advanced medical treatment.
Disclaimer: The information provided is not intended to provide any pharmaceutical or medical device regulatory law advice; instead, all information and content are for general informational purposes only.