In the previous paper, we talked about “cell processed products”, one of the regenerative medicine products defined in the Pharmaceutical and Medical Device Act. In this paper, we will look into another regenerative medicine product, “gene therapy products”.
In the order to enforce the Pharmaceutical and Medical Device Act, gene therapy products are classified into the following three categories:
・Section 1 in Article 2:
- Plasmid vector products
- Virus vector products
- Gene expression products (Except products described in the previous second issue)
What is “gene therapy”?
In the “Guidelines for Clinical Studies of Gene Therapy” published by the Ministry of Health, Labour and Welfare, the definition of gene therapy is “administering genes or transgene cells to the human body for the purpose of disease treatment and prevention”. It means that there are two methods of gene therapy - one method is “to administer genes into the human body”, while the other is “to administer transgene cells into the human body”.
The former method describes a way to administer genes into the human body directly using virus vectors, etc., and is called “in vivo gene therapy”.
On the other hand, the later method describes the manufacture of transgene cells at a cell culturing and processing facility (factory) and administering the manufactured cells into the human body, and is called “ex vivo therapy”.
Also, in the Pharmaceutical and Medical Device Act, only products used for in vivo therapy are categorized as “gene therapy products”, while products used for ex vivo therapy are categorized as “cell processed products”. (Since the gene induction procedure is considered “Processing”, transgene cells are processed products.)
If the final form of a product is “cells” (administering the cells into the human body), the product is categorized as “a gene processed product”. On the other hand, if the final form of a product is “genes (vector)” (administering the genes into the human body), the product is categorized as “a gene therapy product”.
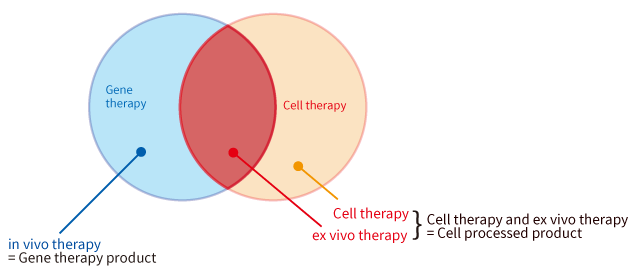
For example, since CAR-T cells used for cancer treatment are T cells inserted with CAR genes, CAR-T cells are categorized as “gene processed products”. Currently gene therapy products are being actively developed in the fields of genetic diseases and cancers.
Genetic diseases are caused by gene mutations or abnormal chromosomes. Gene therapy is mainly used for treatment of “single-gene diseases”. “Single-gene diseases” occur when a single gene stops working properly. Since genetic diseases are caused by the dysfunction of a single gene, gene therapy for genetic diseases is to treat it from the root cause by restoring the dysfunctional gene to normal functions.
Efficacy and Drug Price of Gene Therapy Drug “Zolgensma”.
A gene therapy drug for a single-gene disease, “spinal amyotrophy” was approved in the United States in May 2019.
Spinal amyotrophy is a disease caused by an abnormality of the SMN1 (survival motor neuron 1) gene.
Loss of SMN1 gene function leads to death of the motoneuron cells and promotes muscle weakness and amyotrophia. Spinal amyotrophy is a severe disease. Spinal amyotrophy leads to death, caused by respiratory distress as patients become ultimately unable to maintain muscles required for life activities.
Therefore, the concept of Zolgensma is to prevent the death of motoneuron cells and completely cure the disease by introducing normal SMN1 genes into motoneurons of a spinal amyotrophy patient with virus vector. This Zolgensma treatment is completed through only a one-time intravenous administration of the virus vector.
Zolgensma demonstrated significant clinical effects such as prolonged survival, preservation of motor function, etc. on spinal muscular atrophy patients who are not expected to live more than two years without a ventilator. There were some patients that were able to stand and walk on their own (N Engl J Med 2017; 377: 1713-1722).
Zolgensma drew public attention not only due to its high therapeutic effect but also its high drug price (US$ 2.125 M or approx. JPY 230 M). However, there is data showing that the 10 year medical costs required for treatment of children with severe genetic diseases US$ 4.4 – 5.7 M (costs of ventilators, etc.) If spinal muscular atrophy is completely cured by only a single dose of Zolgensma, this medication would be cost-effective.
“gene therapy products” in the field of oncology include oncolytic viruses.
The oncolytic viruses are designed to grow only in cancer cells, while leaving normal cells intact. Oncolytic viruses infected by cancer cells multiply in the cancer cells, and eventually the viruses destroy the cancer cells and break out. The broken-out oncolytic viruses lyse (shrink) tumors by repeating the cycle of “infecting nearby cancer cells, proliferation, destroying the cancer cells, and breaking out”.
It has been shown that lymphocyte cells may become activated by antigen release when cancer cells are destroyed, and many combination trials with immune checkpoint inhibitors (PD-1 and PD-L1 antibody drugs) have been conducted.
As you can see from above, regenerative medicine products hold the promise of radically curing severe diseases previously treated only by symptomatic care.
Disclaimer: The information provided is not intended to provide any pharmaceutical or medical device regulatory law advice; instead, all information and content are for general informational purposes only.