The Pharmaceutical and Medical Device Act defines regenerative medicine products as two types of products: “Cell processing products” and “Gene therapy products”.
We explained what gene therapy products are in the previous paper. In this paper, we will look into “Gene therapy products” again along with the introduction of approved products.
> Regenerative Medicine Column:What is Gene Therapy Products?(This will open in a new window.)
What is a "gene"?
Genes are sometimes considered as DNA. However, this is not strictly true. DNA and RNA are acidic substances found in the nucleus and named nucleic acids (nucleins) which were discovered by Friedrich Mischa in 1869. The basic structure is similar, and nucleotides binding with base, pentasaccharide and phosphate is the basic unit.
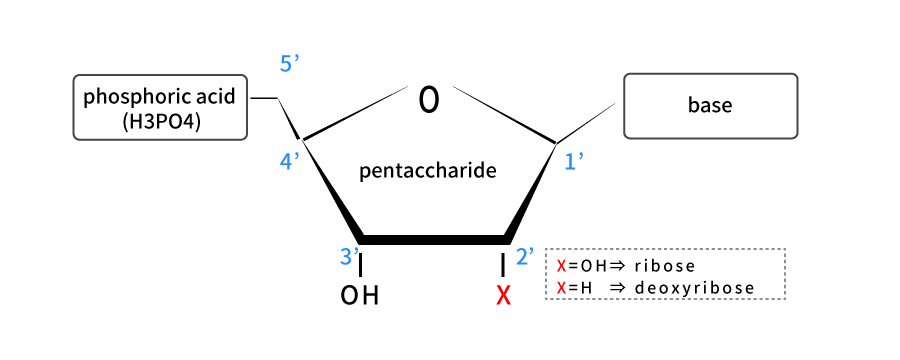
Pentasaccharide of nucleotides making up DNA has hydrogen atom (-H) bonded to 2' carbon, while pentasaccharide of nucleotides making up RNA has a hydroxyl (-OH) bonded to it. Pentasaccharide structure with hydroxyl attached is called ribose, which is an acronym for RNA.
On the other hand, pentasaccharide with no oxygen atoms which means hydrogen atoms bonded to it is called deoxyribose, and it is an acronym for DNA. Pentasaccharide is attached to four bases: adenine (A), guanine (G), cytosine (C) and thymine (T) in DNA, and A, G, C, and uracil (U) instead of T in RNA.
Four types of nucleotides having a single base each are linked together to form a "base sequence" (DNA), and this sequence (a sentence written with four letters) creates genetic information.
As mentioned above, DNA contains genetic information required for living organisms.
However, the substances working in living organisms (cells) are not DNA. They are proteins. The basic unit of proteins is the amino acid, and each protein is determined by the sequence (and combination) of 20 different amino acids. The sequence of amino acids is determined by the DNA base sequence.
It means that DNA is a blueprint for proteins. Among the base sequence recorded by DNA, the part that determines the sequence of these amino acids is called "gene”. The entire base sequence of DNA of an organism is called "genome," of which "genes" are considered to be only a few percent.
Products for gene therapy
Let's take a look at some of regenerative medicine products that have been approved for the purpose of gene therapy. Gene therapies are divided into two types: “in vivo therapy” in which genes are administered into the body for the purpose of treating or preventing the disease, and “ex vivo therapy” in which gene-introduced cells are administered. The Pharmaceutical and Medical Device Act defines only products performing in vivo gene therapy as gene therapy products. (For details, please check the paper of "What are gene therapy products?”)
In addition, in the Pharmaceutical Affairs Law, gene therapy products are classified into three categories such as "1. Plasmid vector products", "2.Virus vector products" and "3. Gene expression products”.
Currently, there are two approved products for the gene therapy: Collategene developed by AnGes, Inc. and Zolgensma by Novartis Pharma K.K. Collategene is classified as a plasmid vector product, while Zolgensma is classified as a viral vector product.
Collategene (generic name: Beperminogene Perplasmid) is cyclic plasmid DNA that expresses human Hepatocyte Growth Factor (hHGF) for the purpose of improving ulcers and rest pain in chronic arterial occlusive disease (atherosclerosis obliterans and Buerger's disease). This product is composed of 5,181 base pairs including the hHGF gene, and is expected to improve ischemic conditions by causing angiogenesis through the production and secretion of HGF.
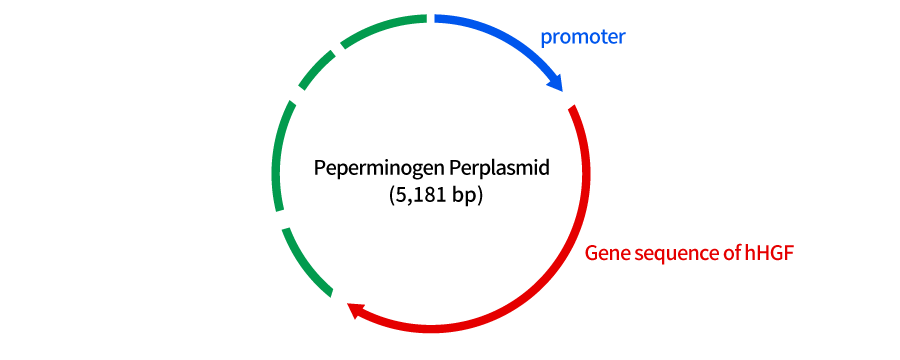
A part of this plasmid DNA contains the gene that expresses hHGF (A part of the DNA has the gene that expresses the protein of interest (hHGF)). When this DNA is injected into the muscle and the gene is transcribed, and production of HGF in the body is expected.
Zolgensma (generic name: onasemnogene abeparvovec) is a regenerative medicine product to treat Spinal Muscular Atrophy (SMA) (including those who have no clinical signs but are expected to develop SMA based on the genetic tests).
SMA is an inherited disease and is caused by defect in the SMN1 gene, a gene producing the SMN protein that is necessary for the survival of spinal motor neurons.
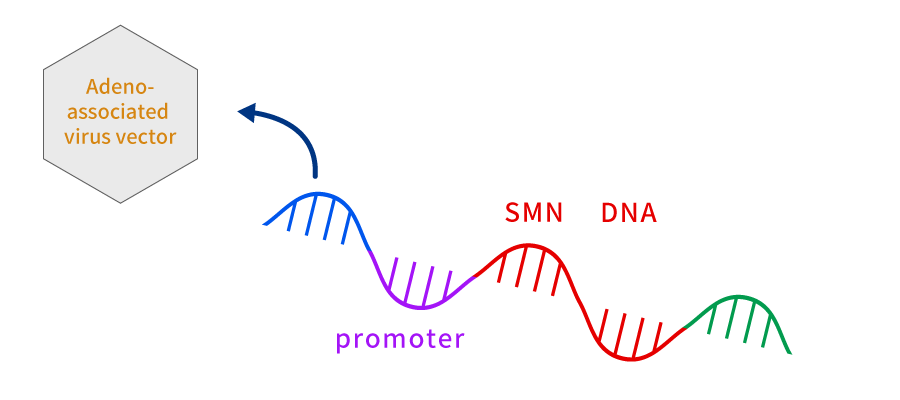
Zolgensma produces an effect by introducing the normal SMN1 gene into Adeno-Associated Virus (AAV) type 9 and being given intravenously (the one that carries the gene into the cell is called a vector. Collategene uses plasmid for gene transfer, so it is called a plasmid vector, and Zolgensma uses virus for gene transfer, so it is called a viral vector.).
> Efficacy and Drug Price of Gene Therapy Drug “Zolgensma”.(This will open in a new window.)
AAV9 vector crosses blood-brain barrier and enters the nucleus of motor neuron, where it releases the transduced SMN1 gene. Thus, the normal SMN1 gene released into the nucleus is read, and SMN protein is continuously produced.
Following the above, various gene therapy products have been under development.
On the other hand, "Ensuring Quality and Safety of Gene Therapy Products” of the Ministry of Health, Labour and Welfare indicates "The implementation and evaluation of studies on individual gene therapy products should be conducted flexibly on a case-by-case basis based on reasonable grounds that reflect current academic progress, taking into account the objectives of this guideline”. The development of gene therapy products needs to be tailored depending on the characteristics of each individual product.
Remedy Group have experienced a large number of clinical trials of gene therapy products, and we can offer support to clients at any development stage.