We have talked about Taiwan as a key player in the Asian clinical trial market in the Asian Newsletter Issue No.20002 (Feb. 2020), showing
- 1.) Large talent pool of well-trained clinical practitioners
- 2.) a small country handling large volumes of clinical trialswell with vast clinical trial experience that comply with international ethical standards
- 3.) a strategic location which is in close proximity to China, Korea and Japan.
In this issue, we will provide deeper insight into the advantages of conducting clinical trials in Taiwan.
Taiwan is always keeping their standards and review process updated with the current trends, by shortening the IRB review timeframes with the cIRB system, shortening the regulatory review timeframe by enacting improvement for the review process guidelines, also constructing harmonization act with advanced Countries.
The potential and capability of Taiwan should not be ignored for successful trials in Asia.
Favorable choice for international clinical strials
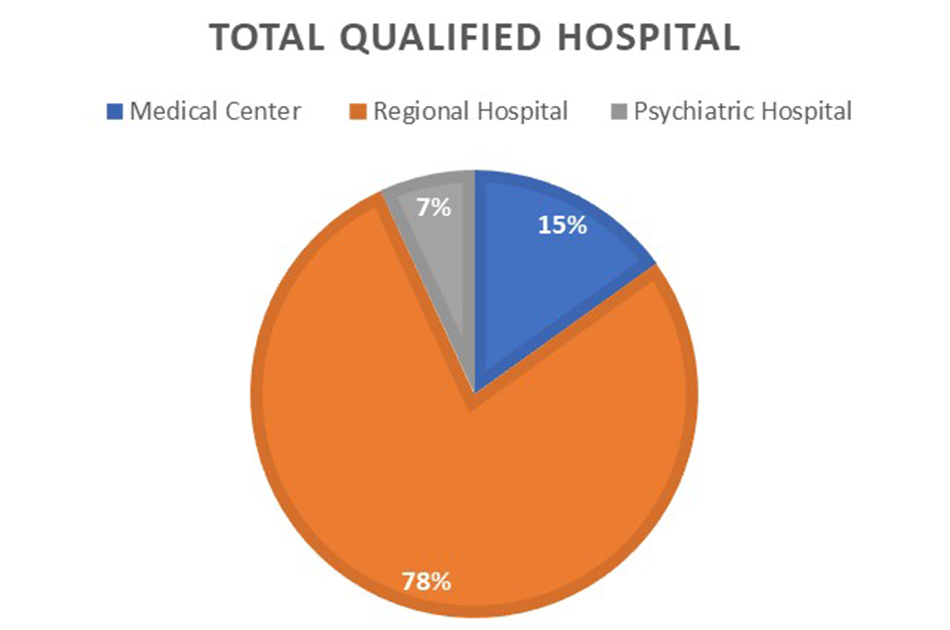
Previously, we have mentioned there are a large talent pool of practitioners, vast trial experience, and international ethical standards that complies with ICH-GCP standards. Let us dig deeper regarding the actual capabilities of Taiwan. In2019, 146 qualified hospitals in Taiwan werecapable ofconducting clinical trials, including 22 medical center class hospital,114 regional teaching hospitals and 10 psychiatric teaching hospitals.……
Full text & latest issue is found in the mail magazine “Global Newsletter”
The “Global Newsletter” provides the latest information pertaining to clinical trials in the Asian region, including timely updates from Local Health Authorities in the regions of coverage and medical advancements of interest.
To subscribe to the Asian Newsletter, please contact us via the following form.